About
Rapid Biomechanics Simulation for Personalized Clinical Design (RAINBOW) is a 'Marie Sklodowska-Curie European Training Network', awarded by the European Union's Horizon 2020 research and innovation programme. The network consists of 7 universities, 1 hospital, 7 industrial partners, located in Denmark, Spain, Luxembourg, England, France and Germany. See details in the list of participants.
The purpose of the network is to educate a total of 15 PhD fellows ('Early Stage Researchers', ESRs) through common dedicated training activities and research stays. The training objective is to balance the scientific training and the transferable skills training to ensure that the ESRs will build skills to enable careers in the academic as well as in the non-academic sectors. The ESRs will be exposed to clinical practice and get close to patients and clinical experts while working both with theory and creating software, design tools, to be used by clinical experts.
The RAINBOW research objective is to realize the full potential of computational medicine and ICT to arrive at patient-specific simulation models that are rapidly set for a particular patient, are easy-to-use by clinical experts and do not require assistance from a technical team.
This is pursued by way of the following Work Packages and objectives.
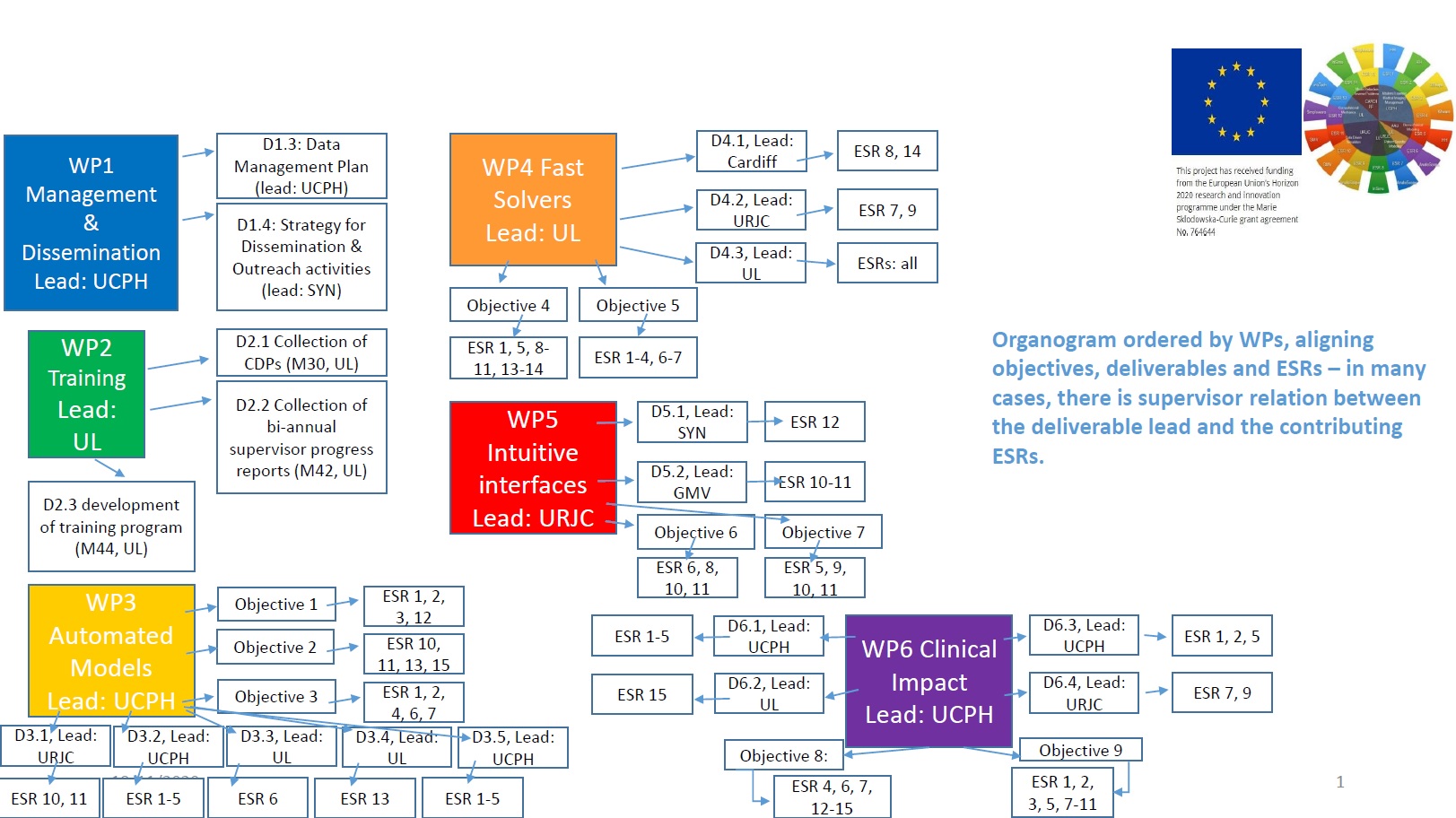
Work packages and objectives
-
WP 'Automated Models':
Taking as input the raw data of an individual subject (e.g. medical images), we will design methods that turn this raw data into a computer model with minimal user intervention. This will require rethinking the current modelling and dscretization methods for biomechanics, tissue viscoelasticity or fluid dynamics, as they typically rely on explicitly defined anatomical interfaces. The WP addresses these objectives:
O1: Discretization of biomechanics models from medical images, with
minimal or no image segmentation (ESRs 1-3, 12)
O2: Semi-automated model and material estimation from personalized medical images (ESRs 10-11, 13, 15)
O3: Automated refitting and registration of biomechanics models to patient-specific data (ESRs 1, 2, 4, 6, 7) -
WP 'Fast Solvers':
Clinical design will rely on semi-interactive tools that provide clinical experts with fast feed back on their design decisions. This will require research on novel biomechanics representations that are accurate yet compact, together with novel efficient solvers that can exploit such compact representations and allowing for efficient algorithms to search the space of clinical designs. The WP addresses these objectives:
O4: Biomechanics solvers based on model-order reduction techniques
(ESRs 1, 5, 8-11, 13, 14)
O5: Massive parallelization of simulation and optimization solvers
(ESRs 1-4, 6, 7) -
WP 'Intuitive Interfaces':
Clinical design tools will be conceived as assistants to the expert knowledge of clinical experts, not as stand-alone tools. This will require novel visualization and interaction paradigms that will allow experts to define otherwise non-intuitive computational aspects (e.g. boundary conditions, objective functions, constraints, degrees of freedom) in an intuitive biomechanics-related manner. The WP addresses these objectives:
O6: Interaction techniques for intuitive, direct manipulation of anatomical
data (ESRs 6, 8, 10, 11)
O7: Intuitive interfaces for design-space specification and exploration
(ESRs 5, 9-11) -
WP ‘Clinical Impact’:
Some of the scientific contributions will be implemented as general-purpose software solutions in the context of existing freely available simulation platforms. Others will be integrated, tested and demonstrated on application-specific software solutions. The WP addresses these objectives:
O8: General-purpose clinical modelling and simulation software solutions (ESRs 4, 6, 7, 12-15)
O9: Application-specific clinical design solutions (ESRs 1-3, 5, 7-11)